https://www.ucl.ac.uk/news/2021/nov/morning-exposure-deep-red-light-improves-declining-eyesight
a once a week three-minute exposure to deep red light could be done
In studying the effects of deep red light in humans, researchers built on their previous findings in mice, bumblebees and fruit flies, which all found significant improvements in the function of the retina’s photoreceptors when their eyes were exposed to 670 nanometre (long wavelength) deep red light.
https://www.sciencedirect.com/science/article/pii/S2666469023000015
The respiratory chain in mitochondria contains many cytochromes, which act as chromophores due to their absorption peaks in the visible and NIR spectral regions. CCO is the terminal enzyme in the electron transport chain in the mitochondria and is also known as complex IV. Many published studies have revealed the role of this complex as an acceptor and transducer of signals activated by light in the red and NIR spectral range [9].
670nm 9LED Deep Red Light Flashlight Against Deteriorating Eyesight Red Torch
it's cheap and takes three weeks to get it from China. hahahah. It's actually from Chunyi Lin's home city!!
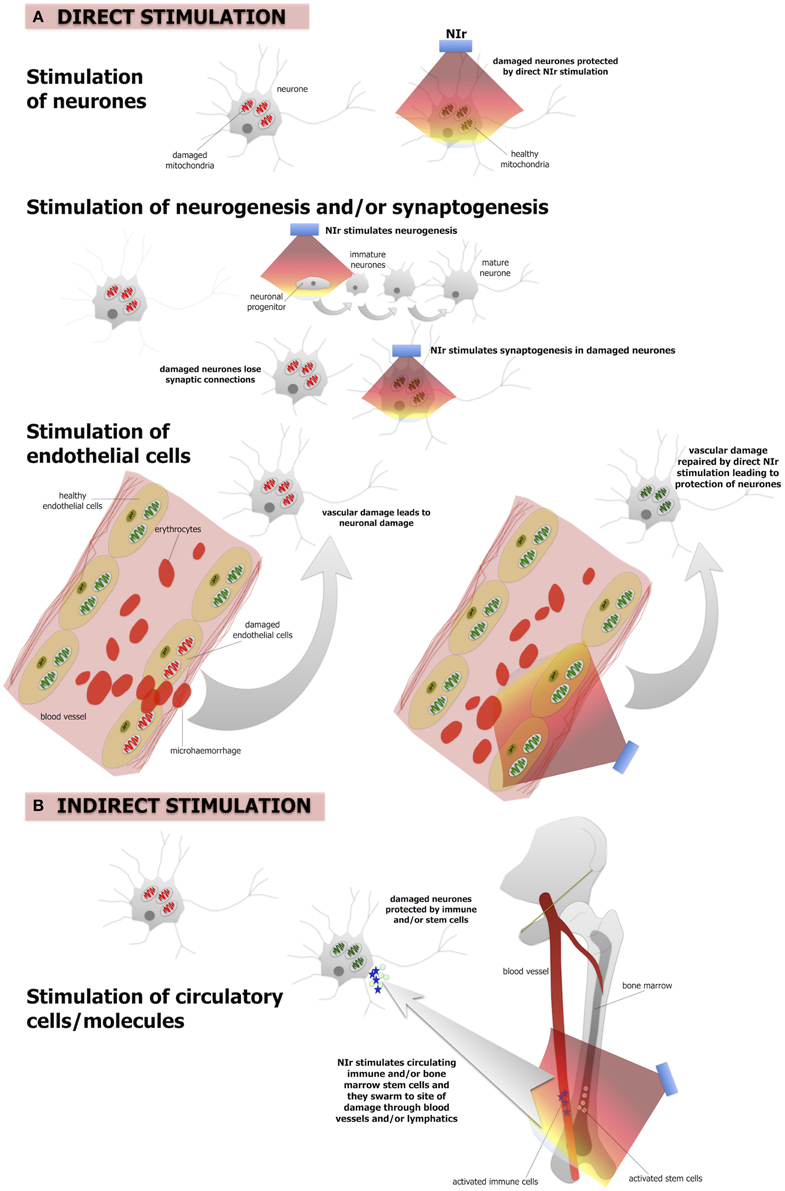
Low-level laser or LED (light emitting diode) therapy using red to infrared light (λ = 600–1070 nm), conflated here to the term “near infrared light” (NIr), is an emerging, putative neuroprotective treatment that is showing promise in several pre-clinical models of disease. For example, NIr has been reported beneficial in animal models of retinal disease (Eells et al., 2004; Natoli et al., 2010, 2013; Albarracin et al., 2013; Begum et al., 2013; Gkotsi et al., 2014), traumatic brain (Ando et al., 2011; Oron et al., 2012; Quirk et al., 2012a; Xuan et al., 2013, 2014, 2015) and optic nerve (Fitzgerald et al., 2010) injury, experimentally-induced stroke (Lapchak et al., 2004; DeTaboada et al., 2006; Oron et al., 2006), familial amyotrophic lateral sclerosis (Moges et al., 2009), multiple sclerosis (Muili et al., 2012), Parkinson's disease (Liang et al., 2008; Whelan et al., 2008; Ying et al., 2008; Shaw et al., 2010; Peoples et al., 2012; Moro et al., 2013, 2014; Purushothuman et al., 2013; Vos et al., 2013; Johnstone et al., 2014a,b; Darlot et al., 2015; El Massri et al., 2015; Reinhart et al., 2015a,b) and Alzheimer's disease (Michalikova et al., 2008; DeTaboada et al., 2011; Grillo et al., 2013; Purushothuman et al., 2014, 2015). In humans, NIr therapy has been reported to improve executive, cognitive, and emotional functions (Barrett and Gonzalez-Lima, 2013; Blanco et al., 2015), together with performance in a range of clinical tests after ischaemic stroke (Lampl et al., 2007; Lapchak, 2010), brain trauma (Naeser et al., 2011, 2014), depression (Schiffer et al., 2009) and in age-related macular degeneration (Merry et al., 2012). The fact that NIr therapy has been reported to be effective in so many different models of disease and in a range of neural systems suggests that it is not a targeted therapy, but instead, acts to mitigate ubiquitous processes relating to cell damage and death. Recent work indicates that NIr is effective in reducing neuronal death induced by apoptosis, but not necrosis (Quirk et al., 2012a). The pathway to apoptosis is likely to involve a critical decline in cellular energy production (Galluzzi et al., 2012), that NIr may help to restore (Hamblin and Demidova, 2006; Liang et al., 2008; Ying et al., 2008; Desmet et al., 2009; Rojas and Gonzalez-Lima, 2011; Chung et al., 2012; Begum et al., 2013; Gkotsi et al., 2014). This mechanism is presumably common to all the above mentioned conditions and is perhaps why NIr therapy has such broad potential applications. In the context of Alzheimer's and Parkinson's disease, although they have distinct initiating causes, both diseases converge on common pathways of inflammation and oxidative stress, mitochondrial dysfunction and neuronal death, indicating that NIr may be beneficial to both through the same protective mechanisms.
https://www.frontiersin.org/articles/10.3389/fnins.2015.00500/full
. There is one sole account of some neuronal damage and negative behavioral outcomes in mice, but this was evident after an exceptionally high power intensity (750 mW/cm2; Ilic et al., 2006), approximately one hundred times higher than the dose required to elicit a therapeutic response (e.g., < 10 mW/cm2).
https://pubmed.ncbi.nlm.nih.gov/24049929/
The photons are absorbed by mitochondrial chromophores in skin cells. Consequently, electron transport, adenosine triphosphate nitric oxide release, blood flow, reactive oxygen species increase, and diverse signaling pathways are activated. Stem cells can be activated, allowing increased tissue repair and healing. In dermatology, LLLT has beneficial effects on wrinkles, acne scars, hypertrophic scars, and healing of burns
https://www.nature.com/articles/s41598-021-02311-1
Time of exposure is critical, as 670 nm light is only effective in the morning. This time dependent effect is likely due to the demonstrated shift in mitochondrial function across the day, and light exposure is likely only effective when synchronised to an aspect of this process.
https://www.sciencedirect.com/science/article/pii/S0014483514000682
Near infra-red (670 nm) is thought to be absorbed by cytochrome c oxidase (COX), a key element in mitochondrial respiration and it has been demonstrated that it improves mitochondrial membrane potentials in aged eyes. It also significantly reduces the impact of experimental pathology and ameliorates age related retinal inflammation. We show ATP decline with ageing in mouse retina and brain.

No comments:
Post a Comment