The COVID-19 vaccine trains immune cells to notice and attack the signature spike protein.
Most of these vaccine strategies are based on the full-length S glycoprotein, which is the major SARS-CoV-2 surface antigen.
Past medical history included systemic arterial hypertension, chronic venous insufficiency, dementia and prostate carcinoma. On January 9, 2021, the man received lipid nanoparticle-formulated, nucleoside-modified RNA vaccine BNT162b2 in a 30 μg dose. On that day and in the following 2 weeks, he presented with no clinical symptoms (Table 1). On day 18, he was admitted to hospital for worsening diarrhea. Since he did not present with any clinical signs of COVID-19, isolation in a specific setting did not occur. Laboratory testing revealed hypochromic anemia and increased creatinine serum levels. Antigen test [spike protein] and polymerase chain reaction (PCR) for SARS-CoV-2 were negative.
The receptor-binding domain of the spike protein of SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2)24 receptors in the nasal cavity, the respiratory tract and other ACE2 receptor locations including intestinal enterocytes,25 leading to endocytosis and release of the viral genome after fusion of membranes.
Another obvious implication is that, compared to viral vectors which must enter the nucleus of a cell, the mRNA is only present in the cytoplasm and it is exposed to active RNA degradation systems (‘RNAase enzymes’) which are the first defense against RNA viruses by reducing the overall degree of translation procedures.Like viral-vector vaccines, Spike proteins and their fragments produced by the infected-cells accommodate on their surface, being rapidly recognized by the host immune system with subsequent production of antibodies. Both vaccine types generate significant neutralizing antibody and virus-specific T cell responses [25, 26]. Specifically, the ability of mRNA and viral vector vaccines to promote intracellular production of Spike protein along with innate immune responses should prime both CD8+ and CD4+ T cells to differentiate into effector and memory subsets by the production of type I interferon [24, 27].
Furthermore, the second dose of these vaccines is associated with an enhancement of the inflammatory response deriving from short-term changes to innate cells like macrophages through a phenomenon called ‘trained immunity’, and/or from activation of memory T cells and B cells generated from the initial injection [24, 27]. It is not entirely clear how these vaccines mobilize the immune response, as well as the durability of protection [24].
The loss of ACE2 receptor activity from the external site of the cellular membrane, as mediated by the interaction between ACE2 and SARS-CoV-2 Spike proteins, leads to less angiotensin II inactivation and less generation of antiotensin1–7 [44, 45]. The imbalance between angiotensin II overactivity and of antiotensin1–7 deficiency may trigger inflammation, thrombosis, and other adverse reactions (Fig. 1) [44, 45]. In this context, it is not clear whether the interaction between free-floating Spike proteins and ACE2 may favor such imbalance and influence the potential adverse events following vaccination (Fig. 1).Generally, these vaccine candidates have demonstrated their ability to induce immune responses and/or neutralizing antibodies [29], [30], [31]. Antibodies binding to the receptor binding domain (RBD) of the Spike protein showed the potential to prevent its interaction with the angiotensin-converting enzyme 2 (ACE2) and neutralize the viruses [29], [30], [31].
But the vaccine - is specifically designed to STAY in the "pre-fusion" stage as attached to the original cell membrane - and NOT the host cell membrane of ACE2.
As noted for the closely related SARS-CoV, SARS-CoV-2 entry into human cells is mediated by ACE2, which acts as a cell membrane receptor
Immunogenicity assessment by measuring spike protein (S1) antigen-binding immunoglobulin (Ig) G in the serum samples obtained at day 25 showed antibody response (8.7 U/ml, reference value <0.8–1.2 U/ml; Roche ECLIA™),
Colonoscopy, in particular, demonstrated an ulcerative lesion of the left colonic flexure, which was histologically diagnosed as ischemic colitis.On day 24, a patient in the same hospital room as our case tested positive for SARS-CoV-2. On day 25, our patient tested SARS-CoV-2 positive by real-time PCR (RT-PCR), with a low cycle threshold (Ct) value indicating high virus load.
Taken together, it appears the patient became infected from the patient in his hospital room.
These results might suggest that the first vaccination induces immunogenicity but not sterile immunity....We examined 9 different tissue samples for known and relevant pathways of virus spreading in the human body (Figure 1). ... We demonstrated viral RNA in nearly all organs examined except for the liver and the olfactory bulb (Figure 1).
Past medical history included systemic arterial hypertension, chronic venous insufficiency, dementia and prostate carcinoma.
Subsequently, the patient’s condition deteriorated under the development of renal insufficiency. On day 24, a patient in the same hospital room as our case tested positive for SARS-CoV-2. On day 25, our patient tested SARS-CoV-2 positive by real-time PCR (RT-PCR), with a low cycle threshold (Ct) value indicating high virus load. On further analysis of the swab sample, there was no evidence for mutant SARS-CoV-2 variants B.1.1.7, B.1.351 or B.1.1.28.1. Taken together, it appears the patient became infected from the patient in his hospital room. Our patient now presented with fever and respiratory discomfort, and lung auscultation displayed crackles. Despite starting supplemental oxygen (2 l per minute) and antibiotic therapy by ceftriaxone, the patient died from acute renal and respiratory failure on the following day.
The kidneys revealed both chronic damage with arteriolosclerosis and interstitial fibrosis, and acute renal failure with hydropic tubular degeneration. The examination of the brain revealed a left parietal pseudocystic tissue necrosis, which was diagnosed as an old infarction area.
However, the patient tested SARS-CoV-2 positive. Both the Ct value measured in nasopharyngeal swab and values measured in formalin-fixed paraffin-embedded autopsy specimens indicate viral load and suggest transmissibility.
We did not find any typical signs of diffuse alveolar damage in the lungs, but we identified extensive acute bronchopneumonia, possibly of bacterial origin. We concluded that the patient died from bronchopneumonia and acute renal failure.
The spike proteins were FROM THE PANDEMIC - not the vaccine.
To evaluate for evidence of previous infection in an individual with history of COVID-19 vaccination, an antibody test specifically evaluating IgM/IgG to the nucleocapsid protein should be used (e.g., for public health surveillance or the diagnosis of MIS-C/MIS-A).
Although he did not present with any COVID-19-specific symptoms, he tested positive for SARS-CoV-2 before he died. Spike protein (S1) antigen-binding showed significant levels for immunoglobulin (Ig) G, while nucleocapsid IgG/IgM was not elicited.
while nucleocapsid IgG/IgM was not elicited
Multiplex RT-PCR analysis targeted 2 independent genes of the SARS-CoV-2-genome (Fluorotype SARS-CoV-2 plus Kit; HAIN/Bruker, Nehren, Germany): RNA-dependent RNA polymerase (Target 1) and nucleopeptide (Target 2).
Elecsys® Anti-SARS-CoV-2 is an immunoassay intended for the qualitative detection of antibodies to SARS-CoV-2 in human serum and plasma.
Immunogenicity assessment by measuring spike protein (S1) antigen-binding immunoglobulin (Ig) G in the serum samples obtained at day 25 showed antibody response (8.7 U/ml, reference value <0.8–1.2 U/ml; Roche ECLIA™),
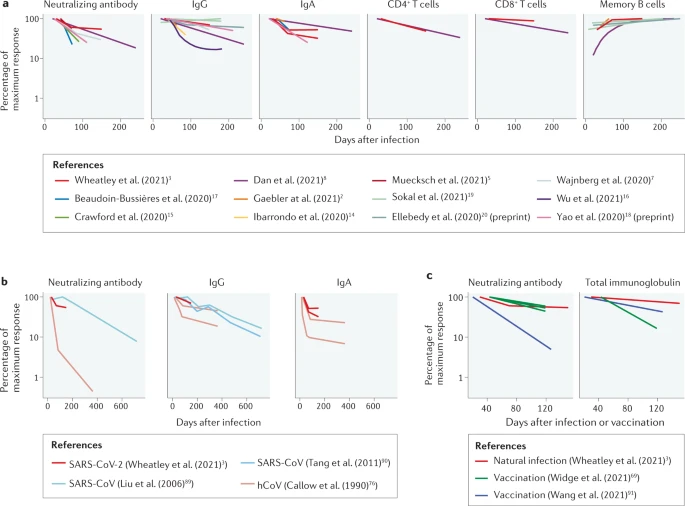
Spike-specific serum IgA levels decay significantly faster than spike-specific IgG, however, the “recall” response for both IgG and IgA (time to peak serum levels following the 2nd / booster dose) is significantly shorter than the primary response.
The amounts that are made after the mRNA is injected are very small and it almost exclusively stays locally. It is nowhere near the amount he was talking about.”
a May 4 commentary in Science Translational Medicine, a publication of the American Association for the Advancement of Science.
The commentary notes that "the spike protein is not released to wander freely through the bloodstream,"
The vaccine shot goes into the muscles for the lymph system...
https://blogs.sciencemag.org/pipeline/archives/2021/05/04/spike-protein-behavior
Compare this, though, to what happens in vaccination. The injection is intramuscular, not into the bloodstream. That’s why a muscle like the deltoid is preferred, because it’s a good target of thicker muscle tissue without any easily hit veins or arteries at the site of injection. The big surface vein in that region is the cephalic vein, and it’s down along where the deltoid and pectoral muscles meet, not high up in the shoulder. In earlier animal model studies of mRNA vaccines, such administration was clearly preferred over a straight i.v. injection; the effects were much stronger. So the muscle cells around the injection are hit by the vaccine (whether mRNA-containing lipid nanoparticles or adenovirus vectors) while a good portion of the remaining dose is in the intercellular fluid and thus drains through the lymphatic system, not the bloodstream. That’s what you want, since the lymph nodes are a major site of immune response. The draining lymph nodes for the deltoid are going to be the deltoid/pectoral ones where those two muscles meet, and the larger axillary lymph nodes down in the armpit on that side.
Now we get to a key difference: when a cell gets the effect of an mRNA nanoparticle or an adenovirus vector, it of course starts to express the Spike protein. But instead of that being assembled into more infectious viral particles, as would happen in a real coronavirus infection, this protein gets moved up to the surface of the cell, where it stays. That’s where it’s presented to the immune system, as an abnormal intruding protein on a cell surface. The Spike protein is not released to wander freely through the bloodstream by itself, because it has a transmembrane anchor region that (as the name implies) leaves it stuck. That’s how it sits in the virus itself, and it does the same in human cells.
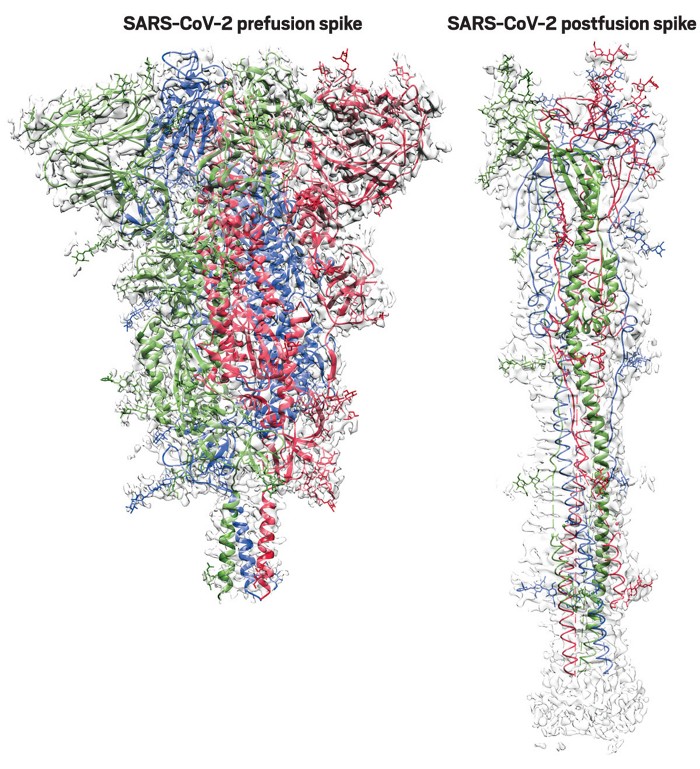
In the Moderna, Pfizer/BioNTech, J&J, and Novavax vaccines, the Spike protein has some proline mutations introduced to try to hold it in its “prefusion” conformation, rather than the shape it adopts when it binds to ACE2. So that should cut down even more on the ability of the Spike protein produced by these vaccines to bind and produce the effects noted in the recent papers. That comes in particularly handy for the Novavax one, since it’s an injection of Spike protein itself, rather than a vaccine that has it produced inside the cells. Notably, the AstraZeneca/Oxford vaccine is producing wild-type Spike (although that’s still going to be membrane-anchored as discussed above!)
https://cen.acs.org/pharmaceuticals/vaccines/tiny-tweak-behind-COVID-19/98/i38
Such antibodies must target the spike protein in its aptly named prefusion conformation. Unfortunately for vaccine developers, spike proteins are liable to spring from their stubby prefusion shape into their elongated postfusion form on a hair trigger.
McLellan discovered that adding two prolines—the most rigid of the 20 amino acids—to a key joint of a vaccine’s spike protein could stabilize the structure’s prefusion shape. This 2P mutation worked in preclinical studies of Graham and Moderna’s MERS vaccine, so they applied it to Moderna’s COVID-19 vaccine.
They homed in on a region toward the top of the spike, a small loop of amino acids that held two coil-like structures called α-helices together.
Blocking the release of that spring should prevent viral fusion, giving the immune system a chance to make antibodies that prevent infection, they theorized.“It is like a spring bent in half,” McLellan says. When the spike protein binds to a human cell, that spring is released, and the two helices and the loop straighten into one long helix that harpoons the human cell and pulls the virus and human membranes close together until they fuse.
adding two prolines in the loop between the two helices clamps the spring together.
https://www.ncbi.nlm.nih.gov/books/NBK27101/
Once the killer T-cell induces the targeted, spike-presenting cell to undergo apoptosis, wouldn’t it likely be engulfed by a macrophage before spikes are released? That way the spikes end up getting digested, rather than floating around freely.
“Cells undergoing programmed cell death are rapidly ingested by nearby phagocytic cells. The phagocytes recognize some change in the cell membrane, most probably the exposure of phosphatidylserine, which is normally found only in the inner leaflet of the membrane. The ingested cell is then completely broken down and digested by the phagocyte without the induction of co-stimulatory proteins. Thus, apoptosis is normally an immunologically ‘quiet’ process; that is, apoptotic cells do not normally contribute to or stimulate immune responses.”
https://www.nature.com/articles/s41586-020-2622-0
We therefore evaluated mRNA formulated in lipid nanoparticles (mRNA–LNP) as a delivery vehicle for MERS-CoV S(2P), and found that transmembrane-anchored MERS-CoV S(2P) mRNA elicited more potent pseudovirus-neutralizing antibody responses than secreted MERS-CoV S(2P) (Extended Data Fig. 1a). Additionally, consistent with protein immunogens, MERS-CoV S(2P) mRNA was more immunogenic than wild-type MERS-CoV S mRNA (Extended Data Fig. 1b).
Within 5 days of the release of the sequence, current good manufacturing practice (cGMP) production of mRNA–LNP encoding the SARS-CoV-2 S(2P) as a transmembrane-anchored protein with the native furin cleavage site (mRNA-1273) was initiated in parallel with preclinical evaluation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8056340/
For example, in Italy, approximately 90% of deaths have occurred in COVID-19 patients aged ≥ 65 years. It has also been demonstrated that underlying diseases such as diabetes, hypertension and obesity increase the risk of severe COVID-19 outcome [17, 18]. Moreover, patients with pre-existing lung problems such as chronic obstructive pulmonary disease (COPD), lung cancer, cystic fibrosis (CF) and severe asthma are more likely to develop severe symptoms of COVID-19 (Table 1).

No comments:
Post a Comment