exercise training reduces the inflammatory potential of DCs [dendritic cells] and macrophages, having significance for inflammation in diverse contexts such as aging, cancer, autoimmunity, and vaccination.
high-intensity tactical training (HITT) or traditional moderate-intensity training (TRAD)
https://www.pnas.org/doi/10.1073/pnas.2406954122
A potential source of inflammation is cell-free DNA (cfDNA)
Acute exercise increased the release of cfDNA from neutrophils, dendritic cells (DCs), and macrophages proportional to exercise intensity. Exercise training reduced cfDNA released in HITT participants but not TRAD and from DCs and macrophages but not neutrophils.
For most participants, training lowered mitochondrial cfDNA at rest, even after detraining.
exercise intensity and training modulated cfDNA release and cytokine responses, contributing to the anti-inflammatory effects of regular exercise.
https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-022-01245-3
Exercise impacts immune homeostasis and leads to rapid and markedly increased levels of cfDNA in blood. Depending on exercise modality, duration, and intensity cfDNA increases 2–20 fold [5,6,7,8]. Even submaximal exercise levels lead to 2–4 fold increases [5], showing a half-life of ~ 15 min [6].
https://pmc.ncbi.nlm.nih.gov/articles/PMC10313937/
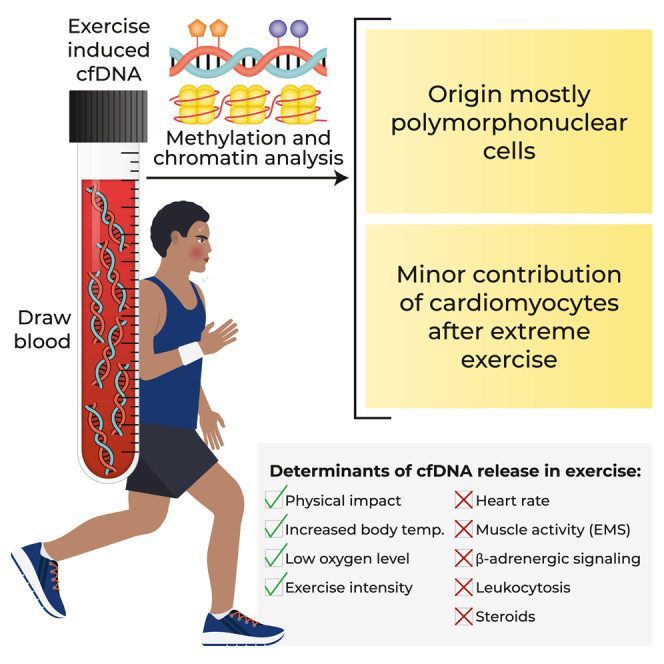
cfDNA in exercise originates mostly in extramedullary polymorphonuclear neutrophils. Strikingly, cardiomyocyte cfDNA concentration increases after a marathon, consistent with elevated troponin levels and indicating low-level, delayed cardiac cell death.
Physical impact, low oxygen levels, and elevated core body temperature contribute to neutrophil cfDNA release, while muscle contraction, increased heart rate, β-adrenergic signaling, or steroid treatment fail to cause elevation of cfDNA. Physical training reduces neutrophil cfDNA release after a standard exercise, revealing an inverse relationship between exercise-induced cfDNA release and training level.
We speculate that the release of cfDNA from neutrophils in exercise relates to the activation of neutrophils in the context of exercise-induced muscle damage.
polymorphonuclear neutrophils as the main source of cfDNA,
Circulating cell-free DNA (cfDNA) fragments are released from dying cells to blood.
The reported increase can be massive (up to 20-fold)16 but persists only for the duration of exercise, with a rapid decline upon cessation of activity.17
exercise-induced oxidative and cytokine stress could potentially drive the release of cfDNA from damaged skeletal muscle or apoptotic leukocytes.14,18
This calculation revealed that neutrophil-derived cfDNA in plasma increased 8- to 98-fold upon exercise, relative to its baseline level of 300 GE/mL. cfDNA from other blood cell types, vascular endothelial cells, and hepatocytes showed a less pronounced (3.5- to 15-fold) elevation in concentration in all exercise protocols, which was less well correlated with the duration of exercise (Figures 1F and S1).
Neutrophil-derived cell-free DNA (cfDNA) is DNA that comes from neutrophils, a type of white blood cell that is part of the innate immune system. Neutrophil-derived cfDNA can be a marker for inflammation and sepsis, and it can also be elevated after exercise.
Elevation of postexercise cfDNA was correlated with both more pronounced low-frequency fatigue (r = −0.52, P = 3.4 × 10−11) and delayed-onset muscle soreness (r = 0.32, P = 0.00019).
https://physoc.onlinelibrary.wiley.com/doi/full/10.1113/EP091986
In this study, we found that cfDNA concentration increases following severe primary and secondary muscle damage after 50 drop jumps.
eccentric lengthening contractions cause more profound sarcomere damage that leads to a more severe inflammatory response (Markus et al., 2021). Drop jumps (DJs) are a very common plyometric exercise used in sports training that are eccentrically biased.
A sarcomere is the basic contractile unit of muscle fiber. Each sarcomere is composed of two main protein filaments—actin and myosin
In healthy individuals, exercise is the primary external variable contributing to increased cfDNA levels (Andreatta et al., 2018; Atamaniuk et al., 2010; Haller et al., 2017).
Many of the published studies have used protocols that require muscles
to perform concentric and eccentric contractions, such as cycling (Tug
et al., 2017), aerobic running (Haller et al., 2017), marathon (Atamaniuk et al., 2008) or lifting exercise (Atamaniuk et al., 2010), and light and heavy resistance training (Andreatta et al., 2018), meaning that simultaneous metabolic stress (accumulation of metabolites, i.e., lactate, H+
ions in muscle cells and altered calcium homeostasis) and mechanical
stress (structural changes involving sarcomere, cytoskeletal and
membrane damage; disruption of excitation–contraction coupling) would
occur. Only one study focused solely on eccentric training, where
high-intensity and low-intensity eccentric cycling exercise was compared
(Mavropalias et al., 2021).
Although the protocol involved eccentric exercise, the participants
performed high-intensity cycling, in which the accumulation of
metabolites would have been present but lower than that of exercise in
most previous studies (Juškevičiūtė et al., 2023; Tug et al., 2017). Hence, the extent to which the increase in cfDNA is caused by mechanically demanding exercise alone is unclear.

No comments:
Post a Comment