The alcohol-induced stimulation of dopamine release in the NAc may require the activity of another category of neuromodulators, endogenous opioid peptides. (For more information on endogenous opioid peptides, see the article by Froehlich, pp. 132–136.) This hypothesis is supported by observations that chemicals that inhibit the actions of endogenous opioid peptides (i.e., opioid peptide antagonists) prevent alcohol’s effects on dopamine release. Opioid peptide antagonists act primarily on a brain area where dopaminergic neurons that extend to the NAc originate. These observations indicate that alcohol stimulates the activity of endogenous opioid peptides, leading indirectly to the activation of dopaminergic neurons. Opioid peptide antagonists would interfere with this process, thereby reducing dopamine release.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6826820/
So we know that alcohol is a diuretic causing loss of electrochemicals and dehydration - by blocking a certain enzyme in the kidney.
We know that alcohol shrinks the pre-frontal cortex and causes neuropathy and dementia.
We know that alcohol causes cancer and hypertension heart problems and sleep apnea and lack of sex drive...
But people really want that opioid induced dopamine bliss.... despite the increase in sympathetic nervous system activation. Quite paradoxical!!
Although numerous studies have attempted to clarify dopamine’s role in alcohol reinforcement by manipulating dopaminergic signal transmission, these investigations do not allow any firm conclusions (for a review, see Di Chiara 1995).
Alcohol misuse is the third leading preventable cause of death in the world. The World
Health Organization currently estimates that 1 in 20 [5%] deaths are directly alcohol related.
To our surprise we did not find that chronic alcohol drinking or abstinence altered protein abundance or pathways associated with inflammation, but rather affected proteins and pathways associated with neurodegeneration and metabolic, cellular organization, protein translation, and molecular transport processes.
A potential molecular mechanism that underlies these effects is that alcohol drinking increased expression of the pro-inflammatory mediators, endothelin-1 (ET-1), COX-2, and PGE2 during alcohol abstinence which may account for the ability of the anti-inflammatory treatments to reduce reinstatement of drinking (Kline and Yamamoto, 2022). Of particular interest, these molecular changes were only observed in the DS, but not the NAc, suggesting that elevated inflammation locally in the DS may drive reinstatement of alcohol drinking after abstinence.
fascinating!!
The dorsal striatum (DS) also plays a major role in action selection behaviors, such as drug seeking and habit formation.
In the DLS [dorsolateral striatum] of female mice that went through acute abstinence, a total of 570 proteins’ abundances were significantly altered, with 311 increased and 259 decreased proteins (Figure 2A).
males had 268 increased and 342 decreased proteins (Figure 2F)
From this search, we identified one neuroinflammatory-related protein that was significantly altered in both females with protracted abstinence and males with acute abstinence, Cluster of Differentiation CD200. CD200 is a key mediator of the innate immune response and neuroinflammation. It has also been shown to decrease in protein abundance in response to alcohol consumption in humans but increase during alcohol abstinence (Chaturvedi et al., 2020).
A variety of dietary polyphenols have been reported to protect against alcoholic liver injury.
Polyphenols can ameliorate alcoholic liver injury by significantly reducing pro-inflammatory factors (Janilkarn-Urena et al. 2023),
This suggests that tea tree essential oil may support gut, liver, and pancreas health, as well as improve immune function.
Our results showed that TTO can promote the secretion of sIgA in the goat intestinal mucosa to maintain intestinal health
So Tea Tree oil is a kind of alcohol exorcism of the body. hahahaha
Furthermore, in recently detoxified participants with AUD [alcohol use disorder], in vivo dopamine D2 and D3 receptor availability appears to be reduced, which may be a predisposing factor or the result of a neuroadaptive process influencing drug-induced DA release.
So alcohol damages the dopamine release receptors....
heavy drinkers are defined as persons who consume five or more drinks on any day in men (or at least 15 drinks per week)
https://karger.com/nps/article/82/6/319/869806
I poured out the fifth beer - so I guess technically I did not binge drink on St. Patrick's day!
Boileau et al. [19] (2003) conducted the first human study revealing a DA release in the nucleus accumbens of social drinkers after an oral alcohol challenge. Most of the following studies were able to replicate this striatal DA release after oral or intravenous alcohol administration – measured indirectly via pre-to-post reduction of radiotracer binding to D2 receptors, which is assumed to be caused by increasing competition between the radiotracer with endogenous DA for binding to the DA receptor during the second PET scan [21‒26].
Whereas most studies reported a significant DA release in striatal regions [19, 21‒26, 30], only one study reported a significant DA release in both the prefrontal and striatal regions [27]. However, in four studies, no DA releasing effect of alcohol administration was reported [28, 29, 31, 32]. Two of these studies focused their investigation on the extrastriatal (mostly prefrontal) brain regions [28, 29, 33‒35] instead of the striatal regions, which may have contributed to these results. In any case, the strongest effects were observed in the ventral striatum/nucleus accumbens [21‒23, 25, 30], which is consistent with findings from animal studies [18].
https://www.mdpi.com/2076-3425/14/5/484
Cholinergic interneurons (CIN) are a major source of acetylcholine (ACh) in the nucleus accumbens (NAc) and have recently garnered attention as being a critical cellular component mediating the behavioral response to rewarding stimuli. Under normal conditions, these interneurons display a tonic activity and are hence often referred to as “tonically active neurons” (reviewed in [1]); however, in the presence of rewarding stimuli, such as cocaine, these neurons ramp up their firing patterns to facilitate reward collection [2,3]. In addition, CIN activity facilitates dopamine release through the activation of dopamine varicosities—specialized sites along dopaminergic axons in which neurotransmitter release occurs—and enhances the motivation to obtain a reward [4,5,6,7]. In the NAc, as compared to GABAergic medium spiny neurons (MSNs; >95%), the number of CIN is small (1–3%); however, with their extensive dendritic and axonal arborizations, the CIN acts as a major regulator of GABAergic synaptic transmission in the NAc [8,9].
.............
The significant findings of this study include (a) A significant increase in the firing rate of CIN in the NAcSh of C57BL/6J mice engaged in binge alcohol consumption compared to those in the sucrose group. This increase was assessed by observing calcium transient events in CIN during alcohol/sucrose consumption. (b) A significant rise in the amplitude of calcium transients, indicating the occurrence of calcium signals in phasic bursts, as evidenced by peak calcium transient activity during alcohol/sucrose consumption. Our findings suggest that CIN may play an important role in binge alcohol consumption.
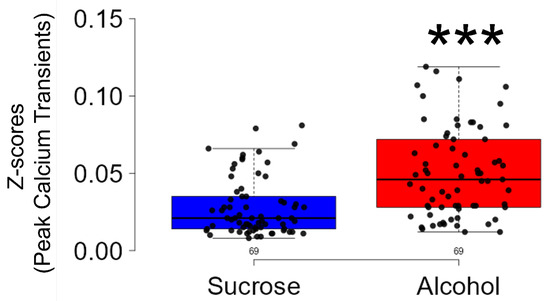 |
| Our investigation revealed a significant rise in both the frequency and amplitude of calcium transients recorded during periods of binge alcohol consumption compared to sucrose intake. |
Strong evidence suggests that NAc is the terminal output center of the mesolimbic dopamine system, which is comprised mainly of the rostral dopaminergic projection from the ventral tegmental area (VTA) to the NAc [33]. Although the activity of dopaminergic systems in the VTA is instrumental in alcohol-related dopamine release in the NAc [33], direct and indirect evidence strongly suggest that this increase in dopamine levels might be attributed to localized phenomena. Specifically, research highlights the role of CIN within the NAc in modulating dopamine release and contributing to reward and addiction mechanisms [11,12,13,42,43,44]. Indeed, while the burst firing in the CIN causes an increase, its inhibition reduces the frequency of dopamine transients in the NAc [4]. In addition, an increase in dopamine efflux in the NAc after the rewarding stimuli, including cocaine, is regulated by CIN [7]. While alcohol is a rewarding substance and CIN regulates dopamine release in the NAc, inducing pleasure in response to the rewarding stimuli, the relationship between binge alcohol consumption and in vivo real-time activity of CIN in the NAcSh has not been investigated.We used in vivo real-time imaging of a genetically encoded calcium indicator (GCaMP6s) to examine CIN activity during voluntary binge alcohol consumption. In vivo calcium imaging allows for the targeted study of neurons in freely behaving animals, linking neural activity with behavior [20,45,46,47].
Moreover, this effect is specific to alcohol because the frequency and amplitude of these neurons were significantly lower during the consumption of sucrose, a natural reinforcer. Although we have not explored the neuroanatomical target downstream of CIN, it is possible that CIN interact with GABAergic MSNs in the NAcSh to affect drinking. These findings strongly implicate CIN as a potential therapeutic target for AUD, indicating its crucial role in modulating binge alcohol consumption.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4813419/
For example, acute binge intoxication is associated with hypothalamically driven increases in blood cortisol, norepinephrine [adrenaline], and sex steroid metabolite levels.
North Americans consume 50% more alcohol than the global average
Dopaminergic neurons in the ventral tegmental area (VTA) are directly activated by alcohol12,13; these dopamine (DA) neurons project to the medium spiny neurons of the ventral striatum (VS), including those of the nucleus accumbens (NAcc) that express dopamine D2 receptors (D2Rs).14 Animal studies have shown that this sharp increase of DA in the VS underlies the initial positively reinforcing effects of alcohol:
alcohol also binds at allosteric modulation sites of gamma amino butyric acid (GABA) receptors; this binding is associated with prolonged chloride channel openings and inhibition of postsynaptic cells.16 GABAergic inhibition within cognitive, emotion regulation, and motivational circuits throughout the cortex and midbrain have been linked to the sedative and negatively reinforcing effects of alcohol.17 Ultimately, the negatively reinforcing effects of alcohol consumption that are associated with GABAergic activity might also be encoded as positively valenced by influencing dopaminergic transmission in the VTA during rewarding processes.
Animal models of acute intoxication indicate that this glucocorticoid release facilitates VS reward activation.24 These stimulatory effects are potentially enhanced by the SAM system activation that results from alcohol’s effects on both the VS and PVN. Specifically, CRF release by the PVN induces excitatory signals to the sympathetic ganglia that synapse with the adrenal medulla.25 This excitatory signal causes the release of acetylcholine in the adrenal medulla, which triggers the release of noradrenaline (NA) into the bloodstream.
Binge Alcohol Consumption Sensitizes Reward Pathways by Altering Stress Regulation System Function
alcohol-induced allostatic overload in mesocortical and mesolimbic pathways may be perpetuated by overactivation of the HPA axis, SAM system, and HPG axis in an attempt to adjust to the physiological load and facilitate neurobehavioral adaptations to adapt to a new set point. Specifically, glucocorticoids secreted via alcohol-induced hypothalamic activation modify reward-related behaviors by stimulating mesencephalic dopaminergic transmission and increasing NE levels in the prefrontal cortex (PFC).24

No comments:
Post a Comment